Research
Supramolecular Chemistry
Traditional molecular chemistry is largely concerned with the synthesis and study of molecules linked by covalent bonds between atoms. However, there is another entire area of chemistry, often impinging on nanometre scale assemblies, that transcends the chemistry of the covalent bond. This is termed supramolecular chemistry and it involves the study of systems bonded by a multitude of non-covalent interactions, particularly hydrogen bonding, π-π stacking, and metal-ligand dative bonds. Many of these kinds of interactions are difficult to control yet their importance and potential is mind blowing. For example, in biochemistry Nature relies heavily on just these interactions to fold proteins into their active conformations and, crucially, it is hydrogen bonding (base pairing) and π-π stacking that give DNA its characteristic double helical shape.1
Molecular Sensors
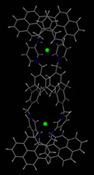
Our work encompasses many aspects of supramolecular chemistry from the nature of individual interactions (particularly in the solid state) to their incorporation and use in functioning molecular devices, particularly in applications such as the design and synthesis of molecular sensors for anions (e.g. environmental pollutants). To take just one example, a complex molecular device based on a calixarene (shown on the right) is capable of selectively recognising and binding a two halide anions entirely through non-covalent interactions (NH···X and CH···X hydrogen bonds) and photochemically signalling that binding via the appended pyrene units.2-5
Supramolecular Gels

Gels comprise a liquid trapped by a highly porous network of nanometre-scale fibres. As well as being fascinating because of their nanoscale structure, recent work has shown that the highly porous, partially ordered network in gels, coupled with their formation by spontaneous self-organization gives them tremendous technological possibilities, for example as selective sorbents or uptake agents, in the controlled formation of highly porous polymers and in the synthesis and support of catalytic nanoparticles. We have discovered an extremely simple, readily prepared series of rigid bis(urea) building blocks in which gelation occurs to give gels via a hierarchical series of self-organization steps strongly influenced (both positively and negatively) by metal salts. The intrinsic ability of bis(ureas) to aggregate via NH∙∙∙O=C hydrogen bonded interactions is modulated and can be switched on and of by reversible coordination interactions to metal cations and hydrogen bonding to conjugate anions. The resulting gels and the consequent nanostructuring of a wide variety of metal salts offers interesting technological possibilities. An SEM image of a dried gel showing chiral helical fibres derived from a chiral gelator is shown right.6, 7
The Molecular Solid State: Pharmaceuticals, Hydrates and Understanding Crystal Packing

A major concern in molecular materials science is the control and understanding of how molecules interact with one another to produce a crystalline solid. The form of the solid packing in pharmaceuticals, for example, can dramatically affect a drug’s bioavailability and processing characteristics and is a huge issue in patent law. Our group is very interested in understanding the factors governing the crystallisation of molecular solids. We have a particular emphasis on ‘weird’ crystals with more than one symmetry-independent molecule (see our Z’ page). We are also very much concerned with polymorphism, co-crystals (particularly hydrates), solid state ‘mechanochemical’ reactions and the interface between crystallisation and gelation.
References
- J. W. Steed and J. L. Atwood, ‘Supramolecular Chemistry’, J. Wiley & Sons: Chichester, 3rd Ed. 2021.
- K. J. Wallace, W. J. Belcher, D. R. Turner, K. F. Syed, and J. W. Steed, “Slow anion exchange, conformational equilibria, and fluorescent sensing in Venus flytrap aminopyridinium-based anion hosts”, J. Am. Chem. Soc., 2003, 125, 9699.
- J. Zhang, A. M. Bond, J. Belcher, K. J. Wallace, and J. W. Steed, “Electrochemical studies on the modular podand 1,3,5-tris(3- ((ferrocenylmethyl)amino)pyridiniumyl)-2,4,6-triethylbenzene hexafluorophosphate in conventional solvents and ionic liquids”, J. Phys. Chem. B, 2003, 107, 5777.
- M. H. Filby, T. D. Humphries, D. R. Turner, R. Kataky, J. Kruusma, and J. W. Steed, “Modular Assembly of a Preorganised, Ditopic Receptor for Dicarboxylates”, Chem. Commun., 2006, 156.
- D. R. Turner, E. C. Spencer, J. A. K. Howard, D. A. Tocher, and J. W. Steed, “A modular, self-assembled, separated ion pair binding system”, Chem. Commun, 2004, 1352.
- L. Applegarth, N. Clark, A. C. Richardson, A. D. M. Parker, I. Radosavljevic-Evans, A. E. Goeta, J. A. K. Howard, and J. W. Steed, “Modular nanometer-scale structuring of gel fibres by sequential self-organization”, Chem. Commun, 2005, 5423.
- C. E. Stanley, N. Clarke, K. M. Anderson, J. P. Lenthall, and J. W. Steed, “Anion Binding Inhibition of the Formation of a Helical Hydrogel”, Chem. Commun., 2006, 3199-3201.






